Which Is A Characteristic Of Animals That Are In The Same Food Web?

A food web is the natural interconnection of nutrient chains and a graphical representation of what-eats-what in an ecological community. Another proper name for food web is consumer-resources system. Ecologists tin broadly lump all life forms into one of two categories called trophic levels: 1) the autotrophs, and two) the heterotrophs. To maintain their bodies, grow, develop, and to reproduce, autotrophs produce organic affair from inorganic substances, including both minerals and gases such as carbon dioxide. These chemic reactions crave energy, which mainly comes from the Lord's day and largely past photosynthesis, although a very small amount comes from bioelectrogenesis in wetlands,[1] and mineral electron donors in hydrothermal vents and hot springs. These trophic levels are not binary, but form a gradient that includes complete autotrophs, which obtain their sole source of carbon from the atmosphere, mixotrophs (such equally carnivorous plants), which are autotrophic organisms that partially obtain organic affair from sources other than the atmosphere, and consummate heterotrophs that must feed to obtain organic matter.
The linkages in a food spider web illustrate the feeding pathways, such as where heterotrophs obtain organic affair past feeding on autotrophs and other heterotrophs. The food web is a simplified illustration of the various methods of feeding that links an ecosystem into a unified system of exchange. There are different kinds of feeding relations that can be roughly divided into herbivory, carnivory, scavenging and parasitism. Some of the organic matter eaten by heterotrophs, such as sugars, provides energy. Autotrophs and heterotrophs come in all sizes, from microscopic to many tonnes - from cyanobacteria to giant redwoods, and from viruses and bdellovibrio to blue whales.
Charles Elton pioneered the concept of food cycles, food chains, and food size in his classical 1927 book "Animal Ecology"; Elton's 'food cycle' was replaced by 'food spider web' in a subsequent ecological text. Elton organized species into functional groups, which was the basis for Raymond Lindeman's classic and landmark paper in 1942 on trophic dynamics. Lindeman emphasized the important role of decomposer organisms in a trophic system of classification. The notion of a food web has a historical foothold in the writings of Charles Darwin and his terminology, including an "entangled banking concern", "web of life", "spider web of complex relations", and in reference to the decomposition actions of earthworms he talked most "the continued move of the particles of earth". Even earlier, in 1768 John Bruckner described nature every bit "i continued web of life".
Nutrient webs are express representations of real ecosystems equally they necessarily aggregate many species into trophic species, which are functional groups of species that have the same predators and prey in a food spider web. Ecologists employ these simplifications in quantitative (or mathematical representation) models of trophic or consumer-resources systems dynamics. Using these models they can mensurate and test for generalized patterns in the structure of real food web networks. Ecologists accept identified not-random properties in the topological structure of nutrient webs. Published examples that are used in meta analysis are of variable quality with omissions. Notwithstanding, the number of empirical studies on community webs is on the rise and the mathematical handling of food webs using network theory had identified patterns that are common to all. Scaling laws, for instance, predict a relationship betwixt the topology of food web predator-prey linkages and levels of species richness.
Taxonomy of a food web [edit]

A simplified food web illustrating a 3 trophic food chain (producers-herbivores-carnivores) linked to decomposers. The motility of mineral nutrients is circadian, whereas the movement of energy is unidirectional and noncyclic. Trophic species are encircled every bit nodes and arrows draw the links.[2] [three]
Nutrient webs are the road-maps through Darwin's famous 'entangled bank' and have a long history in ecology. Like maps of unfamiliar basis, nutrient webs appear bewilderingly circuitous. They were often published to make merely that point. Yet recent studies have shown that food webs from a wide range of terrestrial, freshwater, and marine communities share a remarkable listing of patterns.[iv] : 669
Links in food webs map the feeding connections (who eats whom) in an ecological community. Nutrient cycle is an obsolete term that is synonymous with nutrient spider web. Ecologists can broadly group all life forms into one of two trophic layers, the autotrophs and the heterotrophs. Autotrophs produce more biomass energy, either chemically without the sun'south energy or by capturing the sun's free energy in photosynthesis, than they use during metabolic respiration. Heterotrophs swallow rather than produce biomass energy equally they metabolize, grow, and add to levels of secondary production. A food web depicts a collection of polyphagous heterotrophic consumers that network and cycle the catamenia of energy and nutrients from a productive base of self-feeding autotrophs.[iv] [v] [6]
The base or basal species in a food web are those species without casualty and tin include autotrophs or saprophytic detritivores (i.e., the community of decomposers in soil, biofilms, and periphyton). Feeding connections in the spider web are chosen trophic links. The number of trophic links per consumer is a measure out of nutrient web connectance. Food chains are nested inside the trophic links of food webs. Nutrient chains are linear (noncyclic) feeding pathways that trace monophagous consumers from a base species up to the top consumer, which is usually a larger predatory carnivore.[7] [8] [nine]
| External video | |
|---|---|
| |
Linkages connect to nodes in a nutrient web, which are aggregates of biological taxa called trophic species. Trophic species are functional groups that accept the same predators and prey in a food web. Common examples of an aggregated node in a nutrient web might include parasites, microbes, decomposers, saprotrophs, consumers, or predators, each containing many species in a spider web that can otherwise be connected to other trophic species.[ten] [11]
Trophic levels [edit]
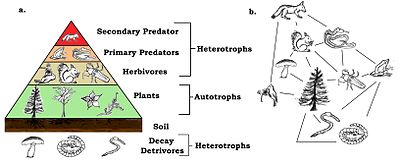
A trophic pyramid (a) and a simplified community food web (b) illustrating ecological relations among creatures that are typical of a northern Boreal terrestrial ecosystem. The trophic pyramid roughly represents the biomass (usually measured equally total dry-weight) at each level. Plants generally have the greatest biomass. Names of trophic categories are shown to the right of the pyramid. Some ecosystems, such as many wetlands, do non organize as a strict pyramid, because aquatic plants are not equally productive as long-lived terrestrial plants such as trees. Ecological trophic pyramids are typically one of three kinds: one) pyramid of numbers, 2) pyramid of biomass, or iii) pyramid of energy.[5]
Nutrient webs have trophic levels and positions. Basal species, such as plants, form the starting time level and are the resource limited species that feed on no other living creature in the spider web. Basal species tin can be autotrophs or detritivores, including "decomposing organic material and its associated microorganisms which we defined as detritus, micro-inorganic material and associated microorganisms (MIP), and tracheophyte cloth."[12] : 94 Most autotrophs capture the sun'south energy in chlorophyll, but some autotrophs (the chemolithotrophs) obtain free energy by the chemical oxidation of inorganic compounds and tin can grow in nighttime environments, such as the sulfur bacterium Thiobacillus, which lives in hot sulfur springs. The height level has tiptop (or apex) predators which no other species kills direct for its food resource needs. The intermediate levels are filled with omnivores that feed on more than one trophic level and cause energy to menstruation through a number of food pathways starting from a basal species.[xiii]
In the simplest scheme, the first trophic level (level 1) is plants, then herbivores (level 2), and and so carnivores (level 3). The trophic level is equal to one more than the concatenation length, which is the number of links connecting to the base. The base of the food concatenation (primary producers or detritivores) is fix at nix.[4] [14] Ecologists identify feeding relations and organize species into trophic species through extensive gut content analysis of different species. The technique has been improved through the use of stable isotopes to better trace energy period through the spider web.[15] It was in one case thought that omnivory was rare, merely recent evidence suggests otherwise. This realization has made trophic classifications more than complex.[16]
Trophic dynamics and multitrophic interactions [edit]
The trophic level concept was introduced in a historical landmark newspaper on trophic dynamics in 1942 by Raymond L. Lindeman. The basis of trophic dynamics is the transfer of energy from one part of the ecosystem to another.[xiv] [17] The trophic dynamic concept has served as a useful quantitative heuristic, just it has several major limitations including the precision by which an organism tin be allocated to a specific trophic level. Omnivores, for instance, are non restricted to whatever single level. Yet, recent research has found that discrete trophic levels do be, only "above the herbivore trophic level, food webs are better characterized as a tangled web of omnivores."[16]
A primal question in the trophic dynamic literature is the nature of control and regulation over resource and production. Ecologists apply simplified i trophic position food chain models (producer, carnivore, decomposer). Using these models, ecologists have tested various types of ecological control mechanisms. For example, herbivores generally have an abundance of vegetative resources, which meant that their populations were largely controlled or regulated past predators. This is known every bit the top-downwardly hypothesis or 'green-world' hypothesis. Alternatively to the top-down hypothesis, not all plant material is edible and the nutritional quality or antiherbivore defenses of plants (structural and chemic) suggests a lesser-upwardly grade of regulation or control.[18] [19] [20] Recent studies take concluded that both "top-down" and "bottom-up" forces can influence community structure and the strength of the influence is environmentally context dependent.[21] [22] These complex multitrophic interactions involve more than than two trophic levels in a nutrient web.[23] For example, such interactions take been discovered in the context of arbuscular mycorrhizal fungi and aphid herbivores that apply the aforementioned plant species.[24]

Multitrophic interaction: Euphydryas editha taylori larvae sequester defensive compounds from specific types of plants they consume to protect themselves from bird predators
Another case of a multitrophic interaction is a trophic cascade, in which predators help to increase constitute growth and forestall overgrazing by suppressing herbivores. Links in a nutrient-spider web illustrate direct trophic relations among species, simply there are also indirect effects that can alter the abundance, distribution, or biomass in the trophic levels. For example, predators eating herbivores indirectly influence the control and regulation of primary product in plants. Although the predators do not eat the plants directly, they regulate the population of herbivores that are directly linked to constitute trophism. The net effect of straight and indirect relations is called trophic cascades. Trophic cascades are separated into species-level cascades, where only a subset of the nutrient-web dynamic is impacted by a change in population numbers, and customs-level cascades, where a alter in population numbers has a dramatic effect on the entire food-spider web, such equally the distribution of plant biomass.[25]
The field of chemic ecology has elucidated multitrophic interactions that entail the transfer of defensive compounds across multiple trophic levels.[26] For example, certain constitute species in the Castilleja and Plantago genera have been constitute to produce defensive compounds called iridoid glycosides that are sequestered in the tissues of the Taylor's checkerspot butterfly larvae that have developed a tolerance for these compounds and are able to consume the foliage of these plants.[27] [28] These sequestered iridoid glycosides then confer chemical protection against bird predators to the butterfly larvae.[27] [28] Some other example of this sort of multitrophic interaction in plants is the transfer of defensive alkaloids produced by endophytes living within a grass host to a hemiparasitic plant that is also using the grass as a host.[29]
Energy flow and biomass [edit]
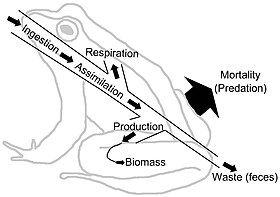
Energy flow diagram of a frog. The frog represents a node in an extended food web. The free energy ingested is utilized for metabolic processes and transformed into biomass. The energy menstruum continues on its path if the frog is ingested past predators, parasites, or every bit a decaying carcass in soil. This free energy menstruation diagram illustrates how energy is lost as it fuels the metabolic process that transform the energy and nutrients into biomass.
The Law of Conservation of Mass dates from Antoine Lavoisier's 1789 discovery that mass is neither created nor destroyed in chemical reactions. In other words, the mass of any i chemical element at the beginning of a reaction volition equal the mass of that element at the end of the reaction.[30] : eleven
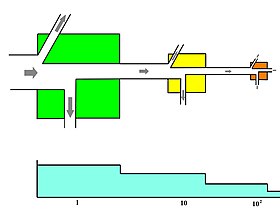
An expanded three link energy food chain (ane. plants, ii. herbivores, 3. carnivores) illustrating the relationship betwixt nutrient flow diagrams and energy transformity. The transformity of energy becomes degraded, dispersed, and macerated from higher quality to bottom quantity as the free energy within a food chain flows from one trophic species into another. Abbreviations: I=input, A=assimilation, R=respiration, NU=not utilized, P=production, B=biomass.[31]
Food webs draw free energy flow via trophic linkages. Energy flow is directional, which contrasts against the cyclic flows of textile through the food web systems.[32] Energy menses "typically includes product, consumption, assimilation, non-assimilation losses (carrion), and respiration (maintenance costs)."[6] : 5 In a very general sense, energy menses (E) can be defined equally the sum of metabolic production (P) and respiration (R), such that Due east=P+R.
Biomass represents stored free energy. However, concentration and quality of nutrients and energy is variable. Many plant fibers, for instance, are boxy to many herbivores leaving grazer community food webs more nutrient limited than detrital food webs where bacteria are able to admission and release the nutrient and free energy stores.[33] [34] "Organisms usually extract energy in the form of carbohydrates, lipids, and proteins. These polymers have a dual role as supplies of energy every bit well equally building blocks; the part that functions as energy supply results in the product of nutrients (and carbon dioxide, water, and rut). Excretion of nutrients is, therefore, basic to metabolism."[34] : 1230–1231 The units in free energy flow webs are typically a measure mass or free energy per one thousand2 per unit of measurement time. Different consumers are going to take different metabolic assimilation efficiencies in their diets. Each trophic level transforms energy into biomass. Energy period diagrams illustrate the rates and efficiency of transfer from one trophic level into another and up through the hierarchy.[35] [36]
It is the case that the biomass of each trophic level decreases from the base of operations of the chain to the top. This is because energy is lost to the environment with each transfer as entropy increases. About lxxx to ninety percent of the energy is expended for the organism's life processes or is lost as oestrus or waste material. Only most ten to twenty percent of the organism's energy is mostly passed to the next organism.[37] The corporeality tin be less than ane percentage in animals consuming less digestible plants, and it can exist every bit high as xl per centum in zooplankton consuming phytoplankton.[38] Graphic representations of the biomass or productivity at each tropic level are called ecological pyramids or trophic pyramids. The transfer of free energy from primary producers to acme consumers can also be characterized by energy catamenia diagrams.[39]
Food chain [edit]
A common metric used to quantify food spider web trophic structure is food chain length. Food chain length is another way of describing nutrient webs as a measure of the number of species encountered as energy or nutrients movement from the plants to elevation predators.[40] : 269 In that location are dissimilar ways of calculating food chain length depending on what parameters of the food web dynamic are existence considered: connectance, energy, or interaction.[forty] In its simplest grade, the length of a concatenation is the number of links between a trophic consumer and the base of the web. The mean chain length of an entire web is the arithmetic boilerplate of the lengths of all chains in a food web.[41] [13]
In a uncomplicated predator-prey example, a deer is one pace removed from the plants it eats (chain length = 1) and a wolf that eats the deer is ii steps removed from the plants (chain length = 2). The relative amount or strength of influence that these parameters take on the nutrient web address questions about:
- the identity or existence of a few dominant species (called strong interactors or keystone species)
- the total number of species and food-chain length (including many weak interactors) and
- how community structure, function and stability is determined.[42] [43]
Ecological pyramids [edit]
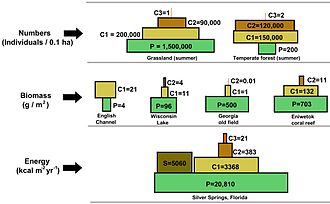
Illustration of a range of ecological pyramids, including top pyramid of numbers, eye pyramid of biomass, and lesser pyramid of energy. The terrestrial forest (summer) and the English Channel ecosystems exhibit inverted pyramids.Notation: trophic levels are not drawn to scale and the pyramid of numbers excludes microorganisms and soil animals. Abbreviations: P=Producers, C1=Primary consumers, C2=Secondary consumers, C3=Tertiary consumers, S=Saprotrophs.[5]
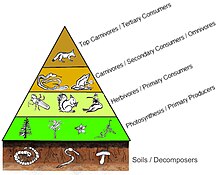
A four level trophic pyramid sitting on a layer of soil and its community of decomposers.
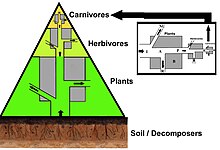
A iii layer trophic pyramid linked to the biomass and energy menstruation concepts.
In a pyramid of numbers, the number of consumers at each level decreases significantly, so that a unmarried top consumer, (due east.g., a polar acquit or a man), will be supported by a much larger number of split producers. At that place is ordinarily a maximum of four or five links in a nutrient concatenation, although food bondage in aquatic ecosystems are more frequently longer than those on state. Somewhen, all the energy in a nutrient concatenation is dispersed as oestrus.[5]
Ecological pyramids identify the main producers at the base. They can draw dissimilar numerical properties of ecosystems, including numbers of individuals per unit of area, biomass (g/mtwo), and energy (one thousand cal m−2 year−one). The emergent pyramidal arrangement of trophic levels with amounts of energy transfer decreasing every bit species become farther removed from the source of production is one of several patterns that is repeated among the planets ecosystems.[three] [4] [44] The size of each level in the pyramid generally represents biomass, which can exist measured as the dry weight of an organism.[45] Autotrophs may have the highest global proportion of biomass, simply they are closely rivaled or surpassed by microbes.[46] [47]
Pyramid structure can vary across ecosystems and beyond time. In some instances biomass pyramids can be inverted. This pattern is frequently identified in aquatic and coral reef ecosystems. The blueprint of biomass inversion is attributed to different sizes of producers. Aquatic communities are often dominated by producers that are smaller than the consumers that have high growth rates. Aquatic producers, such as planktonic algae or aquatic plants, lack the large accumulation of secondary growth equally exists in the woody trees of terrestrial ecosystems. However, they are able to reproduce quickly enough to back up a larger biomass of grazers. This inverts the pyramid. Master consumers have longer lifespans and slower growth rates that accumulates more biomass than the producers they eat. Phytoplankton alive merely a few days, whereas the zooplankton eating the phytoplankton live for several weeks and the fish eating the zooplankton live for several consecutive years.[48] Aquatic predators besides tend to accept a lower expiry rate than the smaller consumers, which contributes to the inverted pyramidal pattern. Population structure, migration rates, and environmental refuge for prey are other possible causes for pyramids with biomass inverted. Energy pyramids, however, will always accept an upright pyramid shape if all sources of food energy are included and this is dictated past the second law of thermodynamics.[five] [49]
Cloth flux and recycling [edit]
Many of the Globe'due south elements and minerals (or mineral nutrients) are contained inside the tissues and diets of organisms. Hence, mineral and nutrient cycles trace nutrient spider web energy pathways. Ecologists employ stoichiometry to analyze the ratios of the chief elements plant in all organisms: carbon (C), nitrogen (N), phosphorus (P). There is a large transitional difference betwixt many terrestrial and aquatic systems equally C:P and C:Due north ratios are much college in terrestrial systems while N:P ratios are equal between the two systems.[50] [51] [52] Mineral nutrients are the material resources that organisms need for growth, development, and vitality. Nutrient webs depict the pathways of mineral nutrient cycling as they flow through organisms.[5] [17] Near of the primary product in an ecosystem is non consumed, simply is recycled by detritus back into useful nutrients.[53] Many of the Earth's microorganisms are involved in the formation of minerals in a process called biomineralization.[54] [55] [56] Leaner that live in detrital sediments create and cycle nutrients and biominerals.[57] Food web models and food cycles have traditionally been treated separately, but there is a strong functional connection between the 2 in terms of stability, flux, sources, sinks, and recycling of mineral nutrients.[58] [59]
Kinds of food webs [edit]
Nutrient webs are necessarily aggregated and only illustrate a tiny portion of the complexity of existent ecosystems. For example, the number of species on the planet are probable in the general lodge of 107, over 95% of these species consist of microbes and invertebrates, and relatively few have been named or classified by taxonomists.[lx] [61] [62] Information technology is explicitly understood that natural systems are 'sloppy' and that food spider web trophic positions simplify the complexity of real systems that sometimes overemphasize many rare interactions. Most studies focus on the larger influences where the bulk of energy transfer occurs.[xviii] "These omissions and problems are causes for concern, but on present bear witness practice not nowadays insurmountable difficulties."[4] : 669
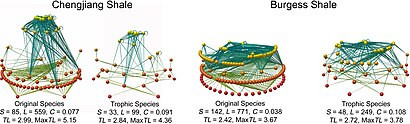
Paleoecological studies can reconstruct fossil food-webs and trophic levels. Primary producers form the base of operations (red spheres), predators at peak (xanthous spheres), the lines represent feeding links. Original food-webs (left) are simplified (right) by aggregating groups feeding on common prey into coarser grained trophic species.[63]
There are unlike kinds or categories of food webs:
- Source spider web - one or more node(south), all of their predators, all the nutrient these predators eat, and so on.
- Sink spider web - i or more node(s), all of their prey, all the nutrient that these prey consume, and so on.
- Community (or connection) web - a group of nodes and all the connections of who eats whom.
- Energy flow spider web - quantified fluxes of free energy between nodes along links betwixt a resource and a consumer.[4] [45]
- Paleoecological spider web - a web that reconstructs ecosystems from the fossil record.[63]
- Functional web - emphasizes the functional significance of certain connections having strong interaction forcefulness and greater bearing on community organisation, more and so than energy flow pathways. Functional webs have compartments, which are sub-groups in the larger network where there are different densities and strengths of interaction.[43] [64] Functional webs emphasize that "the importance of each population in maintaining the integrity of a community is reflected in its influence on the growth rates of other populations."[45] : 511
Within these categories, food webs tin can exist further organized co-ordinate to the different kinds of ecosystems being investigated. For example, homo food webs, agricultural food webs, detrital food webs, marine nutrient webs, aquatic food webs, soil food webs, Arctic (or polar) food webs, terrestrial nutrient webs, and microbial food webs. These characterizations stalk from the ecosystem concept, which assumes that the phenomena under investigation (interactions and feedback loops) are sufficient to explain patterns within boundaries, such as the edge of a woods, an isle, a shoreline, or another pronounced physical characteristic.[65] [66] [67]
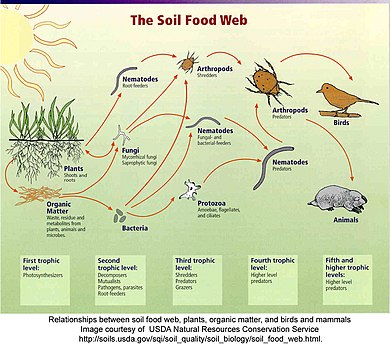
An analogy of a soil nutrient web.
Detrital web [edit]
In a detrital web, plant and creature matter is broken downwardly by decomposers, e.g., leaner and fungi, and moves to detritivores and so carnivores.[68] In that location are ofttimes relationships between the detrital web and the grazing spider web. Mushrooms produced past decomposers in the detrital web become a food source for deer, squirrels, and mice in the grazing spider web. Earthworms eaten by robins are detritivores consuming decaying leaves.[69]
"Detritus can be broadly defined every bit whatever form of non-living organic thing, including different types of plant tissue (e.k. leaf litter, dead wood, aquatic macrophytes, algae), animate being tissue (carrion), dead microbes, faeces (manure, dung, faecal pellets, guano, frass), too as products secreted, excreted or exuded from organisms (e.one thousand. extra-cellular polymers, nectar, root exudates and leachates, dissolved organic matter, extra-cellular matrix, mucilage). The relative importance of these forms of detritus, in terms of origin, size and chemic limerick, varies across ecosystems."[53] : 585
Quantitative nutrient webs [edit]
Ecologists collect data on trophic levels and nutrient webs to statistically model and mathematically summate parameters, such equally those used in other kinds of network analysis (e.k., graph theory), to study emergent patterns and backdrop shared among ecosystems. At that place are different ecological dimensions that can exist mapped to create more complicated nutrient webs, including: species composition (blazon of species), richness (number of species), biomass (the dry weight of plants and animals), productivity (rates of conversion of free energy and nutrients into growth), and stability (food webs over fourth dimension). A nutrient spider web diagram illustrating species composition shows how modify in a unmarried species can direct and indirectly influence many others. Microcosm studies are used to simplify food web inquiry into semi-isolated units such as small springs, decaying logs, and laboratory experiments using organisms that reproduce quickly, such as daphnia feeding on algae grown under controlled environments in jars of water.[42] [lxx]
While the complexity of existent food webs connections are difficult to decipher, ecologists take plant mathematical models on networks an invaluable tool for gaining insight into the structure, stability, and laws of nutrient web behaviours relative to observable outcomes. "Food spider web theory centers around the idea of connectance."[71] : 1648 Quantitative formulas simplify the complexity of food spider web structure. The number of trophic links (tL), for instance, is converted into a connectance value:
- ,
where, Southward(Due south-1)/ii is the maximum number of binary connections amidst Southward species.[71] "Connectance (C) is the fraction of all possible links that are realized (L/S2) and represents a standard mensurate of food web complexity..."[72] : 12913 The distance (d) between every species pair in a web is averaged to compute the mean distance betwixt all nodes in a web (D)[72] and multiplied by the total number of links (L) to obtain link-density (LD), which is influenced by scale dependent variables such every bit species richness. These formulas are the basis for comparing and investigating the nature of not-random patterns in the structure of nutrient spider web networks among many different types of ecosystems.[72] [73]
Scaling laws, complexity, chaos, and pattern correlates are common features attributed to food web structure.[74] [75]
Complexity and stability [edit]
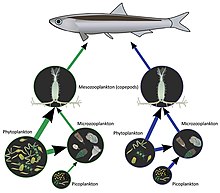
A simplified version of a food web in the Gulf of Naples in eutrophic (Green) and oligotrophic (Blue) summer conditions. In the Green system state, both copepods and microzooplankton exert a potent grazing pressure on phytoplankton, while in the Blue land, copepods increment their predation over microzooplankton, which in plow shifts its predation from phytoplankton to bacterial plankton or picoplankton. These trophic mechanisms stabilize the delivery of organic matter from copepods to fish.
Food webs are extremely complex. Complexity is a measure of an increasing number of permutations and it is also a metaphorical term that conveys the mental intractability or limits apropos unlimited algorithmic possibilities. In food web terminology, complexity is a production of the number of species and connectance.[76] [77] [78] Connectance is "the fraction of all possible links that are realized in a network".[79] : 12917 These concepts were derived and stimulated through the proposition that complication leads to stability in nutrient webs, such equally increasing the number of trophic levels in more than species rich ecosystems. This hypothesis was challenged through mathematical models suggesting otherwise, but subsequent studies take shown that the premise holds in real systems.[76] [73]
At different levels in the hierarchy of life, such as the stability of a food web, "the same overall structure is maintained in spite of an ongoing flow and change of components."[80] : 476 The farther a living organization (east.g., ecosystem) sways from equilibrium, the greater its complexity.[80] Complexity has multiple meanings in the life sciences and in the public sphere that misfile its application every bit a precise term for analytical purposes in scientific discipline.[78] [81] Complexity in the life sciences (or biocomplexity) is defined by the "properties emerging from the interplay of behavioral, biological, concrete, and social interactions that affect, sustain, or are modified by living organisms, including humans".[82] : 1018
Several concepts have emerged from the study of complication in food webs. Complexity explains many principals pertaining to self-arrangement, non-linearity, interaction, cybernetic feedback, discontinuity, emergence, and stability in food webs. Nestedness, for instance, is divers as "a pattern of interaction in which specialists interact with species that form perfect subsets of the species with which generalists collaborate",[83] : 575 "—that is, the nutrition of the most specialized species is a subset of the diet of the side by side more generalized species, and its diet a subset of the adjacent more generalized, so on."[84] Until recently, information technology was idea that nutrient webs had little nested structure, but empirical evidence shows that many published webs take nested subwebs in their assembly.[85]
Food webs are complex networks. Equally networks, they exhibit similar structural properties and mathematical laws that have been used to describe other circuitous systems, such every bit small world and scale costless backdrop. The pocket-size world attribute refers to the many loosely connected nodes, non-random dense clustering of a few nodes (i.eastward., trophic or keystone species in environmental), and small path length compared to a regular lattice.[79] [86] "Ecological networks, peculiarly mutualistic networks, are generally very heterogeneous, consisting of areas with sparse links among species and distinct areas of tightly linked species. These regions of high link density are ofttimes referred to as cliques, hubs, compartments, cohesive sub-groups, or modules...Inside food webs, especially in aquatic systems, nestedness appears to be related to trunk size because the diets of smaller predators tend to exist nested subsets of those of larger predators (Woodward & Warren 2007; YvonDurocher et al. 2008), and phylogenetic constraints, whereby related taxa are nested based on their common evolutionary history, are also axiomatic (Cattin et al. 2004)."[87] : 257 "Compartments in food webs are subgroups of taxa in which many strong interactions occur within the subgroups and few weak interactions occur between the subgroups. Theoretically, compartments increase the stability in networks, such equally nutrient webs."[64]
Food webs are also complex in the way that they change in scale, seasonally, and geographically. The components of food webs, including organisms and mineral nutrients, cross the thresholds of ecosystem boundaries. This has led to the concept or area of study known every bit cantankerous-purlieus subsidy.[65] [66] "This leads to anomalies, such as food web calculations determining that an ecosystem tin can support one half of a acme carnivore, without specifying which end."[67] Nonetheless, real differences in structure and part take been identified when comparing different kinds of ecological nutrient webs, such as terrestrial vs. aquatic food webs.[88]
History of food webs [edit]
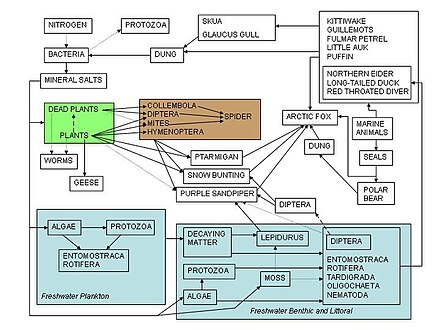
Victor Summerhayes and Charles Elton's 1923 nutrient web of Comport Island (Arrows point to an organism being consumed by another organism).
Food webs serve as a framework to help ecologists organize the circuitous network of interactions among species observed in nature and around the earth. 1 of the primeval descriptions of a food concatenation was described past a medieval Afro-Arab scholar named Al-Jahiz: "All animals, in short, cannot exist without food, neither tin the hunting animal escape being hunted in his plough."[89] : 143 The earliest graphical depiction of a food web was by Lorenzo Camerano in 1880, followed independently by those of Pierce and colleagues in 1912 and Victor Shelford in 1913.[90] [91] Two nutrient webs about herring were produced by Victor Summerhayes and Charles Elton[92] and Alister Hardy[93] in 1923 and 1924. Charles Elton subsequently pioneered the concept of nutrient cycles, food chains, and nutrient size in his classical 1927 book "Animal Ecology"; Elton'south 'food cycle' was replaced past 'food web' in a subsequent ecological text.[94] Afterwards Charles Elton's use of nutrient webs in his 1927 synthesis,[95] they became a central concept in the field of ecology. Elton[94] organized species into functional groups, which formed the ground for the trophic system of classification in Raymond Lindeman's classic and landmark paper in 1942 on trophic dynamics.[17] [43] [96] The notion of a food web has a historical foothold in the writings of Charles Darwin and his terminology, including an "entangled bank", "web of life", "web of circuitous relations", and in reference to the decomposition actions of earthworms he talked about "the continued movement of the particles of globe". Even before, in 1768 John Bruckner described nature as "ane continued web of life".[4] [97] [98] [99]
Interest in nutrient webs increased after Robert Paine'southward experimental and descriptive study of intertidal shores[100] suggesting that nutrient spider web complexity was key to maintaining species diverseness and ecological stability. Many theoretical ecologists, including Sir Robert May[101] and Stuart Pimm,[102] were prompted by this discovery and others to examine the mathematical properties of nutrient webs.
See also [edit]
- Anti-predator adaptation – Defensive feature of prey for selective advantage
- Apex predator – Predator at the top of a food chain
- Aquatic-terrestrial subsidies
- Balance of nature – Superseded ecological theory
- Biodiversity – Variety and variability of life forms
- Biogeochemical wheel – Cycling of substances through biotic and abiotic compartments of Earth
- Consumer–resource interactions
- Ecological network
- Food system – Processes by which nutritional substances are grown, raised, packaged and distributed
- Food web of the San Francisco Estuary
- List of feeding behaviours
- Marine nutrient spider web
- Microbial food web
- Natural environment – All living and non-living things occurring naturally, generally on Earth
- Soil food web
- Tritrophic interactions in plant defense – Ecological interactions
- Trophic ecology of kelp forests – Underwater areas with a high density of kelp
- Trophic mutualism
- Trophic relationships in lakes – Blazon of ecosystem
- Trophic relationships in rivers – Blazon of aquatic ecosystem with flowing freshwater
References [edit]
- ^ Nowak, M. E.; Beulig, F.; von Fischer, J.; Muhr, J.; Küsel, Grand.; Trumbore, S. E. (2015). "Autotrophic fixation of geogenic CO2 by microorganisms contributes to soil organic thing germination and alters isotope signatures in a wetland mofette" (PDF). Biogeosciences. Copernicus Publications (published 2015-12-08). 12 (23): 7169–7183. Bibcode:2015BGeo...12.7169N. doi:x.5194/bg-12-7169-2015 . Retrieved 2019-10-01 .
- ^ Kormondy, E. J. (1996). Concepts of ecology (quaternary ed.). New Jersey: Prentice-Hall. p. 559. ISBN978-0-13-478116-7.
- ^ a b Proulx, South. R.; Promislow, D. E. L.; Phillips, P. C. (2005). "Network thinking in environmental and evolution" (PDF). Trends in Ecology and Evolution. 20 (vi): 345–353. doi:10.1016/j.tree.2005.04.004. PMID 16701391. Archived from the original (PDF) on 2011-08-fifteen.
- ^ a b c d e f k Pimm, S. L.; Lawton, J. H.; Cohen, J. Due east. (1991). "Nutrient web patterns and their consequences" (PDF). Nature. 350 (6320): 669–674. Bibcode:1991Natur.350..669P. doi:10.1038/350669a0. S2CID 4267587. Archived from the original (PDF) on 2010-06-ten.
- ^ a b c d due east f Odum, E. P.; Barrett, G. Due west. (2005). Fundamentals of Ecology (fifth ed.). Brooks/Cole, a part of Cengage Learning. ISBN978-0-534-42066-vi. Archived from the original on 2011-08-20.
- ^ a b Benke, A. C. (2010). "Secondary production". Nature Education Noesis. 1 (8): 5.
- ^ Allesina, S.; Alonso, D.; Pascual, M. (2008). "A general model for food spider web structure" (PDF). Science. 320 (5876): 658–661. Bibcode:2008Sci...320..658A. doi:10.1126/scientific discipline.1156269. PMID 18451301. S2CID 11536563. Archived from the original (PDF) on 2011-09-28.
- ^ Azam, F.; Fenche, T.; Field, J. K.; Gra, J. S.; Meyer-Reil, L. A.; Thingstad, F. (1983). "The ecological role of water-column microbes in the sea" (PDF). Mar. Ecol. Prog. Ser. 10: 257–263. Bibcode:1983MEPS...10..257A. doi:10.3354/meps010257.
- ^ Uroz, South.; Calvarus, C.; Turpault, M.; Frey-Klett, P. (2009). "Mineral weathering by bacteria: ecology, actors and mechanisms" (PDF). Trends in Microbiology. 17 (8): 378–387. doi:10.1016/j.tim.2009.05.004. PMID 19660952. [ permanent dead link ]
- ^ Williams, R. J.; Martinez, N. D. (2000). "Elementary rules yield complex food webs" (PDF). Nature. 404 (6774): 180–183. Bibcode:2000Natur.404..180W. doi:10.1038/35004572. PMID 10724169. S2CID 205004984.
- ^ Mail service, D. M. (2002). "The long and brusque of nutrient chain length" (PDF). Trends in Ecology and Evolution. 17 (6): 269–277. doi:10.1016/S0169-5347(02)02455-two. Archived from the original (PDF) on 2011-07-28.
- ^ Tavares-Cromar, A. F.; Williams, D. D. (1996). "The importance of temporal resolution in nutrient web analysis: Testify from a detritus-based stream" (PDF). Ecological Monographs. 66 (1): 91–113. doi:10.2307/2963482. hdl:1807/768. JSTOR 2963482.
- ^ a b Pimm, S. L. (1979). "The structure of food webs" (PDF). Theoretical Population Biology. 16 (2): 144–158. doi:10.1016/0040-5809(79)90010-8. PMID 538731. Archived from the original (PDF) on 2011-09-27.
- ^ a b Cousins, Southward. (1985-07-04). "Ecologists build pyramids again". New Scientist. 1463: fifty–54.
- ^ McCann, Yard. (2007). "Protecting biostructure". Nature. 446 (7131): 29. Bibcode:2007Natur.446...29M. doi:10.1038/446029a. PMID 17330028. S2CID 4428058.
- ^ a b Thompson, R. M.; Hemberg, Yard.; Starzomski, B. M.; Shurin, J. B. (March 2007). "Trophic levels and trophic tangles: The prevalence of omnivory in existent food webs" (PDF). Ecology. 88 (iii): 612–617. doi:ten.1890/05-1454. PMID 17503589. Archived from the original (PDF) on 2011-08-fifteen.
- ^ a b c Lindeman, R. L. (1942). "The trophic-dynamic aspect of environmental" (PDF). Ecology. 23 (four): 399–417. doi:10.2307/1930126. JSTOR 1930126.
- ^ a b Hairston, N. Yard. (1993). "Cause-effect relationships in energy menstruation, trophic structure, and interspecific interactions" (PDF). The American Naturalist. 142 (3): 379–411. doi:x.1086/285546. hdl:1813/57238. S2CID 55279332. Archived from the original (PDF) on 2011-07-20.
- ^ Fretwell, S. D. (1987). "Food chain dynamics: The central theory of ecology?" (PDF). Oikos. l (3): 291–301. doi:10.2307/3565489. JSTOR 3565489. Archived from the original (PDF) on 2011-07-28.
- ^ Polis, Thousand. A.; Stiff, D. R. (1996). "Food web complexity and community dynamics" (PDF). The American Naturalist. 147 (v): 813–846. doi:10.1086/285880. S2CID 85155900.
- ^ Hoekman, D. (2010). "Turning upwardly the head: Temperature influences the relative importance of top-down and bottom-upwards effects" (PDF). Ecology. 91 (10): 2819–2825. doi:ten.1890/10-0260.i. PMID 21058543.
- ^ Schmitz, O. J. (2008). "Herbivory from individuals to ecosystems". Annual Review of Ecology, Evolution, and Systematics. 39: 133–152. doi:10.1146/annurev.ecolsys.39.110707.173418. S2CID 86686057.
- ^ Tscharntke, T.; Hawkins, B., A., eds. (2002). Multitrophic Level Interactions. Cambridge: Cambridge University Printing. p. 282. ISBN978-0-521-79110-half-dozen.
- ^ Babikova, Zdenka; Gilbert, Lucy; Bruce, Toby; Dewhirst, Sarah; Pickett, John A.; Johnson, David (Apr 2014). "Arbuscular mycorrhizal fungi and aphids interact past changing host plant quality and volatile emission". Functional Environmental. 28 (2). Retrieved 2022-05-02 .
- ^ Polis, G.A.; et al. (2000). "When is a trophic cascade a trophic pour?" (PDF). Trends in Ecology and Evolution. 15 (11): 473–5. doi:10.1016/S0169-5347(00)01971-6. PMID 11050351.
- ^ Tscharntke, Teja; Hawkins, Bradford A. (2002). Multitrophic Level Interactions. Cambridge: Cambridge University Press. pp. 10, 72. ISBN978-0-511-06719-viii.
- ^ a b Haan, Nate L.; Bakker, Jonathan D.; Bowers, M. Deane (14 Jan 2021). "Preference, operation, and chemical defense in an endangered butterfly using novel and ancestral host plants". Scientific Reports. 11 (992). doi:ten.1038/s41598-020-80413-y. Retrieved 2022-05-02 .
- ^ a b Haan, Nate L.; Bakker, Jonathan D.; Bowers, Grand. Deane (May 2018). "Hemiparasites tin can transmit indirect effects from their host plants to herbivores". Ecology. 99 (2). Retrieved 2022-05-02 .
- ^ Lehtonen, Päivi; Helander, Marjo; Flash, Michael; Sporer, Frank; Saikkonen, Kari (12 October 2005). "Transfer of endophyte-origin defensive alkaloids from a grass to a hemiparasitic found". Ecology Letters. 8 (12). doi:x.1111/j.1461-0248.2005.00834.x. Retrieved 2022-05-02 .
- ^ Sterner, R. W.; Small, G. E.; Hood, J. Thou. "The conservation of mass". Nature Education Knowledge. two (ane): 11.
- ^ Odum, H. T. (1988). "Self-organization, transformity, and information". Science. 242 (4882): 1132–1139. Bibcode:1988Sci...242.1132O. doi:10.1126/science.242.4882.1132. JSTOR 1702630. PMID 17799729. S2CID 27517361.
- ^ Odum, Eastward. P. (1968). "Energy flow in ecosystems: A historical review". American Zoologist. eight (i): 11–eighteen. doi:10.1093/icb/8.1.eleven.
- ^ Isle of mann, K. H. (1988). "Product and apply of detritus in various freshwater, estuarine, and littoral marine ecosystems" (PDF). Limnol. Oceanogr. 33 (2): 910–930. doi:10.4319/lo.1988.33.4_part_2.0910. Archived from the original (PDF) on 2012-04-25.
- ^ a b Koijman, S. A. L. K.; Andersen, T.; Koo, B. Due west. (2004). "Dynamic energy budget representations of stoichiometric constraints on population dynamics" (PDF). Ecology. 85 (5): 1230–1243. doi:10.1890/02-0250.
- ^ Anderson, Thou. H.; Beyer, J. East.; Lundberg, P. (2009). "Trophic and private efficiencies of size-structured communities". Proc Biol Sci. 276 (1654): 109–114. doi:10.1098/rspb.2008.0951. PMC2614255. PMID 18782750.
- ^ Benke, A. C. (2011). "Secondary product, quantitative nutrient webs, and trophic position". Nature Educational activity Knowledge. 2 (two): ii.
- ^ Spellman, Frank R. (2008). The Science of H2o: Concepts and Applications. CRC Press. p. 165. ISBN978-1-4200-5544-iii.
- ^ Kent, Michael (2000). Advanced Biological science. Oxford University Press US. p. 511. ISBN978-0-nineteen-914195-1.
- ^ Kent, Michael (2000). Advanced Biology. Oxford University Press US. p. 510. ISBN978-0-19-914195-1.
- ^ a b Post, D. Grand. (1993). "The long and short of food-chain length". Trends in Environmental and Evolution. 17 (6): 269–277. doi:x.1016/S0169-5347(02)02455-ii.
- ^ Odum, Eastward. P.; Barrett, K. W. (2005). Fundamentals of environmental. Brooks Cole. p. 598. ISBN978-0-534-42066-half dozen. [ permanent dead link ]
- ^ a b Worm, B.; Duffy, J.E. (2003). "Biodiversity, productivity and stability in existent food webs". Trends in Environmental and Evolution. 18 (12): 628–632. doi:ten.1016/j.tree.2003.09.003.
- ^ a b c Paine, R. T. (1980). "Nutrient webs: Linkage, interaction force and community infrastructure". Journal of Animal Environmental. 49 (three): 666–685. doi:10.2307/4220. JSTOR 4220. S2CID 55981512.
- ^ Raffaelli, D. (2002). "From Elton to mathematics and back again". Science. 296 (5570): 1035–1037. doi:10.1126/scientific discipline.1072080. PMID 12004106. S2CID 177263265.
- ^ a b c Rickleffs, Robert, E. (1996). The Economy of Nature. University of Chicago Press. p. 678. ISBN978-0-7167-3847-3.
- ^ Whitman, W. B.; Coleman, D. C.; Wieb, Westward. J. (1998). "Prokaryotes: The unseen majority". Proc. Natl. Acad. Sci. USA. 95 (12): 6578–83. Bibcode:1998PNAS...95.6578W. doi:10.1073/pnas.95.12.6578. PMC33863. PMID 9618454.
- ^ Groombridge, B.; Jenkins, M. (2002). Earth Atlas of Biodiversity: Earth'southward Living Resources in the 21st Century. World Conservation Monitoring Heart, United Nations Surround Programme. ISBN978-0-520-23668-4.
- ^ Spellman, Frank R. (2008). The Scientific discipline of Water: Concepts and Applications. CRC Press. p. 167. ISBN978-1-4200-5544-iii.
- ^ Wang, H.; Morrison, W.; Singh, A.; Weiss, H. (2009). "Modeling inverted biomass pyramids and refuges in ecosystems" (PDF). Ecological Modelling. 220 (11): 1376–1382. doi:10.1016/j.ecolmodel.2009.03.005. Archived from the original (PDF) on 2011-10-07.
- ^ Pomeroy, L. R. (1970). "The strategy of mineral cycling". Annual Review of Ecology and Systematics. one: 171–190. doi:10.1146/annurev.es.01.110170.001131. JSTOR 2096770.
- ^ Elser, J. J.; Fagan, W. F.; Donno, R. F.; Dobberfuhl, D. R.; Folarin, A.; Huberty, A.; et al. (2000). "Nutritional constraints in terrestrial and freshwater food webs" (PDF). Nature. 408 (6812): 578–580. Bibcode:2000Natur.408..578E. doi:ten.1038/35046058. PMID 11117743. S2CID 4408787. [ permanent dead link ]
- ^ Koch, P. L.; Fox-Dobbs, K.; Newsom, South. D. "The isotopic ecology of fossil vertebrates and conservation paleobiology". In Nutrition, G. P.; Flessa, Thou. W. (eds.). Conservation paleobiology: Using the past to manage for the future, Paleontological Society short course (PDF). The Paleontological Society Papers. Vol. fifteen. pp. 95–112.
- ^ a b Moore, J. C.; Berlow, E. L.; Coleman, D. C.; de Ruiter, P. C.; Dong, Q.; Hastings, A.; et al. (2004). "Detritus, trophic dynamics and biodiversity". Ecology Letters. seven (7): 584–600. doi:10.1111/j.1461-0248.2004.00606.ten. S2CID 2635427.
- ^ H. A., Lowenstam (1981). "Minerals formed past organisms". Scientific discipline. 211 (4487): 1126–1131. Bibcode:1981Sci...211.1126L. doi:10.1126/science.7008198. JSTOR 1685216. PMID 7008198. S2CID 31036238.
- ^ Warren, L. A.; Kauffman, Yard. E. (2003). "Microbial geoengineers". Scientific discipline. 299 (5609): 1027–1029. doi:ten.1126/science.1072076. JSTOR 3833546. PMID 12586932. S2CID 19993145.
- ^ González-Muñoz, Thou. T.; Rodriguez-Navarro, C.; Martínez-Ruiz, F.; Arias, J. M.; Merroun, M. Fifty.; Rodriguez-Gallego, Chiliad. (2010). "Bacterial biomineralization: new insights from Myxococcus-induced mineral precipitation". Geological Society, London, Special Publications. 336 (1): 31–50. Bibcode:2010GSLSP.336...31G. doi:x.1144/SP336.3. S2CID 130343033.
- ^ Gonzalez-Acosta, B.; Bashan, Y.; Hernandez-Saavedra, N. Y.; Ascencio, F.; De la Cruz-Agüero, One thousand. (2006). "Seasonal seawater temperature as the major determinant for populations of culturable bacteria in the sediments of an intact mangrove in an arid region" (PDF). FEMS Microbiology Environmental. 55 (2): 311–321. doi:10.1111/j.1574-6941.2005.00019.x. PMID 16420638.
- ^ DeAngelis, D. L.; Mulholland, P. J.; Palumbo, A. V.; Steinman, A. D.; Huston, M. A.; Elwood, J. W. (1989). "Food dynamics and food-spider web stability". Annual Review of Environmental and Systematics. 20: 71–95. doi:10.1146/annurev.ecolsys.20.1.71. JSTOR 2097085.
- ^ Twiss, Grand. R.; Campbell, P. Thou. C.; Auclair, J. (1996). "Regeneration, recycling, and trophic transfer of trace metals by microbial nutrient-web organisms in the pelagic surface waters of Lake Erie". Limnology and Oceanography. 41 (7): 1425–1437. Bibcode:1996LimOc..41.1425T. doi:10.4319/lo.1996.41.7.1425.
- ^ May, R. M. (1988). "How many species are there on Globe?" (PDF). Science. 241 (4872): 1441–1449. Bibcode:1988Sci...241.1441M. doi:10.1126/science.241.4872.1441. PMID 17790039. S2CID 34992724. Archived from the original (PDF) on 2013-05-xi. Retrieved 2011-06-13 .
- ^ Beattie, A.; Ehrlich, P. (2010). "The missing link in biodiversity conservation". Science. 328 (5976): 307–308. Bibcode:2010Sci...328..307B. doi:10.1126/science.328.5976.307-c. PMID 20395493.
- ^ Ehrlich, P. R.; Pringle, R. M. (2008). "Colloquium Paper: Where does biodiversity go from here? A grim concern-as-usual forecast and a hopeful portfolio of partial solutions". Proceedings of the National Academy of Sciences. 105 (S1): 11579–11586. Bibcode:2008PNAS..10511579E. doi:10.1073/pnas.0801911105. PMC2556413. PMID 18695214.
- ^ a b Dunne, J. A.; Williams, R. J.; Martinez, North. D.; Wood, R. A.; Erwin, D. H.; Dobson, Andrew P. (2008). "Compilation and Network Analyses of Cambrian Food Webs". PLOS Biology. vi (4): e102. doi:10.1371/journal.pbio.0060102. PMC2689700. PMID 18447582.
- ^ a b Krause, A. E.; Frank, Yard. A.; Mason, D. M.; Ulanowicz, R. E.; Taylor, W. Due west. (2003). "Compartments revealed in food-web structure" (PDF). Nature. 426 (6964): 282–285. Bibcode:2003Natur.426..282K. doi:ten.1038/nature02115. hdl:2027.42/62960. PMID 14628050. S2CID 1752696.
- ^ a b Bormann, F. H.; Likens, G. E. (1967). "Nutrient cycling" (PDF). Science. 155 (3761): 424–429. Bibcode:1967Sci...155..424B. doi:10.1126/science.155.3761.424. PMID 17737551. S2CID 35880562. Archived from the original (PDF) on 2011-09-27.
- ^ a b Polis, G. A.; Anderson, W. B.; Hold, R. D. (1997). "Toward an integration of mural and food spider web environmental: The dynamics of spatially subsidized food webs" (PDF). Annual Review of Ecology and Systematics. 28: 289–316. doi:x.1146/annurev.ecolsys.28.1.289. hdl:1808/817. Archived from the original (PDF) on 2011-x-02.
- ^ a b O'Neil, R. V. (2001). "Is it time to bury the ecosystem concept? (With full military honors, of course!)" (PDF). Environmental. 82 (12): 3275–3284. doi:ten.1890/0012-9658(2001)082[3275:IITTBT]2.0.CO;ii. Archived from the original (PDF) on 2012-04-25.
- ^ Gönenç, I. Ethem; Koutitonsky, Vladimir K.; Rashleigh, Brenda (2007). Assessment of the Fate and Effects of Toxic Agents on Water Resources. Springer. p. 279. ISBN978-ane-4020-5527-0.
- ^ Gil Nonato C. Santos; Alfonso C. Danac; Jorge P. Ocampo (2003). E-Biology Two. Rex Volume Store. p. 58. ISBN978-971-23-3563-1.
- ^ Elser, J.; Hayakawa, K.; Urabe, J. (2001). "Nutrient Limitation Reduces Nutrient Quality for Zooplankton: Daphnia Response to Seston Phosphorus Enrichment". Environmental. 82 (3): 898–903. doi:ten.1890/0012-9658(2001)082[0898:NLRFQF]2.0.CO;2.
- ^ a b Paine, R. T. (1988). "Road maps of interactions or grist for theoretical evolution?" (PDF). Environmental. 69 (6): 1648–1654. doi:ten.2307/1941141. JSTOR 1941141. Archived from the original (PDF) on 2011-07-28.
- ^ a b c Williams, R. J.; Berlow, Due east. L.; Dunne, J. A.; Barabási, A.; Martinez, N. D. (2002). "Two degrees of separation in complex food webs". Proceedings of the National Academy of Sciences. 99 (20): 12913–12916. Bibcode:2002PNAS...9912913W. doi:ten.1073/pnas.192448799. PMC130559. PMID 12235367.
- ^ a b Banasek-Richter, C.; Bersier, 50. L.; Cattin, M.; Baltensperger, R.; Gabriel, J.; Merz, Y.; et al. (2009). "Complexity in quantitative food webs". Ecology. 90 (half dozen): 1470–1477. doi:ten.1890/08-2207.1. hdl:1969.one/178777. PMID 19569361.
- ^ Riede, J. O.; Rall, B. C.; Banasek-Richter, C.; Navarrete, S. A.; Wieters, E. A.; Emmerson, Thousand. C.; et al. (2010). "Scaling of food web properties with diversity and complexity across ecosystems.". In Woodwoard, Thou. (ed.). Advances in Ecological Research (PDF). Vol. 42. Burlington: Academic Press. pp. 139–170. ISBN978-0-12-381363-3.
- ^ Briand, F.; Cohen, J. E. (1987). "Environmental correlates of food chain length" (PDF). Science. 238 (4829): 956–960. Bibcode:1987Sci...238..956B. doi:ten.1126/science.3672136. PMID 3672136. Archived from the original (PDF) on 2012-04-25.
- ^ a b Neutel, A.; Heesterbeek, J. A. P.; de Ruiter, P. D. (2002). "Stability in existent food webs: Weak link in long loops" (PDF). Science. 295 (550): 1120–1123. Bibcode:2002Sci...296.1120N. doi:10.1126/scientific discipline.1068326. hdl:1874/8123. PMID 12004131. S2CID 34331654. Archived from the original (PDF) on 2011-09-28.
- ^ Leveque, C., ed. (2003). Ecology: From ecosystem to biosphere. Scientific discipline Publishers. p. 490. ISBN978-ane-57808-294-0.
- ^ a b Proctor, J. D.; Larson, B. G. H. (2005). "Ecology, complexity, and metaphor". BioScience. 55 (12): 1065–1068. doi:ten.1641/0006-3568(2005)055[1065:ECAM]2.0.CO;two.
- ^ a b Dunne, J. A.; Williams, R. J.; Martinez, North. D. (2002). "Food-spider web structure and network theory: The role of connectance and size". Proceedings of the National Academy of Sciences. 99 (twenty): 12917–12922. Bibcode:2002PNAS...9912917D. doi:10.1073/pnas.192407699. PMC130560. PMID 12235364.
- ^ a b Capra, F. (2007). "Complexity and life". Syst. Res. 24 (5): 475–479. doi:ten.1002/sres.848.
- ^ Peters, R. H. (1988). "Some general problems for ecology illustrated by food spider web theory". Ecology. 69 (6): 1673–1676. doi:10.2307/1941145. JSTOR 1941145.
- ^ Michener, Due west. One thousand.; Baerwald, T. J.; Firth, P.; Palmer, M. A.; Rosenberger, J. L.; Sandlin, East. A.; Zimmerman, H. (2001). "Defining and unraveling biocomplexity". BioScience. 51 (12): 1018–1023. doi:ten.1641/0006-3568(2001)051[1018:daub]2.0.co;two.
- ^ Bascompte, J.; Jordan, P. (2007). "Institute-animal mutualistic networks: The architecture of biodiversity" (PDF). Annu. Rev. Ecol. Evol. Syst. 38: 567–569. doi:x.1146/annurev.ecolsys.38.091206.095818. hdl:10261/40177. Archived from the original (PDF) on 2009-ten-25.
- ^ Montoya, J. M.; Pimm, S. L.; Solé, R. 5. (2006). "Ecological networks and their fragility" (PDF). Nature. 442 (7100): 259–264. Bibcode:2006Natur.442..259M. doi:10.1038/nature04927. PMID 16855581. S2CID 592403. Archived from the original (PDF) on 2010-07-06.
- ^ Michio, K.; Kato, S.; Sakato, Y. (2010). "Food webs are built up with nested subwebs". Ecology. 91 (xi): 3123–3130. doi:10.1890/09-2219.1. PMID 21141173.
- ^ Montoya, J. K.; Solé, R. V. (2002). "Small-scale globe patterns in food webs" (PDF). Journal of Theoretical Biological science. 214 (iii): 405–412. arXiv:cond-mat/0011195. Bibcode:2002JThBi.214..405M. doi:10.1006/jtbi.2001.2460. PMID 11846598. Archived from the original (PDF) on 2011-09-05.
- ^ Montoya, J. M.; Blüthgen, North; Brown, 50.; Dormann, C. F.; Edwards, F.; Figueroa, D.; et al. (2009). "Ecological networks: beyond food webs". Journal of Animal Environmental. 78 (1): 253–269. doi:10.1111/j.1365-2656.2008.01460.x. PMID 19120606.
- ^ Shurin, J. B.; Gruner, D. S.; Hillebrand, H. (2006). "All moisture or dried upward? Real differences between aquatic and terrestrial food webs". Proc. R. Soc. B. 273 (1582): i–9. doi:10.1098/rspb.2005.3377. PMC1560001. PMID 16519227.
- ^ Egerton, F. N. "A history of the ecological sciences, part half-dozen: Arabic language science: Origins and zoological writings" (PDF). Bulletin of the Ecological Society of America. 83 (ii): 142–146.
- ^ Egerton, FN (2007). "Understanding food bondage and food webs, 1700-1970". Message of the Ecological Order of America. 88: fifty–69. doi:10.1890/0012-9623(2007)88[50:UFCAFW]ii.0.CO;2.
- ^ Shelford, Five. (1913). "Animal Communities in Temperate America as Illustrated in the Chicago Region". University of Chicago Press.
- ^ Summerhayes, VS; Elton, CS (1923). "Contributions to the Ecology of Spitsbergen and Bear Island". Periodical of Ecology. eleven (two): 214–286. doi:10.2307/2255864. JSTOR 2255864.
- ^ Hardy, Ac (1924). "The herring in relation to its animate surroundings. Role 1. The food and feeding habits of the herring with special reference to the east declension of England". Fisheries Investigation London Series Two. 7 (3): one–53.
- ^ a b Elton, C. S. (1927). Animal Ecology. London, Britain.: Sidgwick and Jackson. ISBN978-0-226-20639-four.
- ^ Elton CS (1927) Fauna Ecology. Republished 2001. University of Chicago Press.
- ^ Allee, W. C. (1932). Fauna life and social growth. Baltimore: The Williams & Wilkins Company and Associates.
- ^ Stauffer, R. C. (1960). "Ecology in the long manuscript version of Darwin's "Origin of Species" and Linnaeus' "Oeconomy of Nature"". Proc. Am. Philos. Soc. 104 (two): 235–241. JSTOR 985662.
- ^ Darwin, C. R. (1881). The formation of vegetable mould, through the activity of worms, with observations on their habits. London: John Murray.
- ^ Worster, D. (1994). Nature's economy: A history of ecological ideas (2nd ed.). Cambridge Academy Printing. p. 423. ISBN978-0-521-46834-iii.
- ^ Paine, RT (1966). "Food web complication and species diversity". The American Naturalist. 100 (910): 65–75. doi:10.1086/282400. S2CID 85265656.
- ^ May RM (1973) Stability and Complication in Model Ecosystems. Princeton University Press.
- ^ Pimm SL (1982) Nutrient Webs, Chapman & Hall.
Further reading [edit]
- Cohen, Joel E. (1978). Food webs and niche space. Monographs in Population Biology. Vol. 11. Princeton, NJ: Princeton University Press. pp. 15+ane–190. ISBN978-0-691-08202-8. PMID 683203.
- "Aquatic Food Webs". NOAA Education Resources. National Oceanic and Atmospheric Administration.
Source: https://en.wikipedia.org/wiki/Food_web
Posted by: mcelroywitaysen.blogspot.com


0 Response to "Which Is A Characteristic Of Animals That Are In The Same Food Web?"
Post a Comment